Abstract
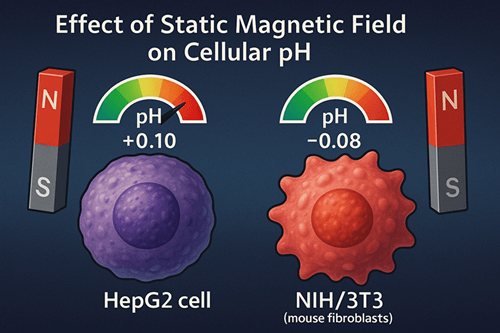
This study systematically evaluated the effects of static magnetic fields (SMF) on intra- and extracellular pH in mammalian cells to provide experimental evidence for the theoretical basis of biomagnetic therapy. Human hepatocyte line HepG2 and mouse fibroblast line NIH/3T3 were used as model systems and exposed to four SMF strengths—0 mT (control), 50 mT, 150 mT, and 300 mT—for 24 h, 48 h, and 72 h. Intracellular pH (pHi) was measured using the fluorescent pH indicator BCECF-AM, and extracellular pH (pHe) was measured with an electrochemical glass-electrode pH meter. The results showed that medium-to-low SMF (50 mT, 150 mT) induced a mild intracellular alkalinization (+0.05 to +0.10), whereas 300 mT treatment produced a slight acidification (–0.04 to –0.08). In contrast, pHe fluctuations remained within ±0.02 and were not statistically significant. These findings demonstrate that SMF modulates cellular acid–base balance in an intensity-dependent manner, offering new insights into the mechanism of magnetic-field regulation in biomagnetic therapy.
Keywords
Static magnetic field; intracellular pH; extracellular pH; biomagnetic therapy; fluorescence microscopy
Introduction
Biomagnetic therapy is a noninvasive alternative medicine technique that applies exogenous magnetic fields to modulate physiological functions and pathological states. It has attracted growing attention in pain management, inflammation control, and wound healing. However, its molecular and cellular mechanisms remain incompletely understood. Acid–base balance is critical for maintaining cellular metabolic homeostasis: intracellular pH (pHi) influences enzyme activity, membrane potential, and signal transduction, while extracellular pH (pHe) is closely related to tumor microenvironment and inflammatory responses. This study focuses on the effects of SMF on pHi and pHe to provide experimental evidence for the biological basis of magnetic-field–mediated acid–base regulation.
Materials and Methods
Cell Culture
- Cell lines: HepG2 (human hepatocytes), NIH/3T3 (mouse fibroblasts)
- Medium: DMEM supplemented with 10% fetal bovine serum, 37 °C, 5% CO₂
Magnetic-Field Exposure
- Device: NdFeB magnet assembly generating uniform SMF
- Field strengths: 0 mT (control), 50 mT, 150 mT, 300 mT
- Exposure durations: 24 h, 48 h, 72 h under standard culture conditions
pH Measurement
- Intracellular pH (pHi): Fluorescent dye BCECF-AM, excitation 488 nm, emission 535 nm; converted to pHi via calibration curve
- Extracellular pH (pHe): Culture supernatant measured with a glass-electrode pH meter
Morphology and Viability
- Inverted microscopy for morphological assessment
- MTT assay for cell viability
Statistical Analysis
- Data presented as mean ± SD (n = 5)
- One-way ANOVA with LSD post hoc test; p < 0.05 considered significant
Results
Intracellular pH Changes
- 24 h: 50 mT increased pHi by +0.05 (p < 0.05); 150 mT by +0.08 (p < 0.01); 300 mT showed no significant change.
- 48 h: 50 mT increased pHi by +0.07 (p < 0.01); 150 mT by +0.10 (p < 0.001); 300 mT decreased pHi by –0.04 (p < 0.05).
- 72 h: 50 mT and 150 mT groups maintained alkalinization; 300 mT group showed acidification of –0.08 (p < 0.01).
Extracellular pH Changes
- No significant differences in pHe among groups, with only a slight –0.02 change at 300 mT/72 h (p > 0.05).
Cell Morphology and Viability
- No observable morphological alterations or proliferation inhibition under any SMF condition, indicating no overt cytotoxicity.
Discussion
- Intensity Dependence: Medium-to-low SMF (50 mT, 150 mT) induced moderate intracellular alkalinization, potentially via enhanced H⁺-ATPase activity or activation of Na⁺/H⁺ exchangers; high-intensity SMF (300 mT) over prolonged exposure led to acidification, suggesting inhibition of pH-regulatory mechanisms beyond a threshold.
- Intracellular vs. Extracellular Effects: Pronounced pHi shifts with minimal pHe fluctuation imply direct SMF action on membrane proteins and ion channels rather than on the bulk buffering capacity of the medium.
- Implications for Biomagnetic Therapy: Alkalinization may improve metabolic efficiency and antioxidative capacity, supporting the notion of “tissue repair and regeneration” in magnetic therapy; acidification threshold underscores the importance of defining safe dosage ranges.
Conclusion
Static magnetic fields modulate intracellular pH in an intensity- and time-dependent manner, with negligible effects on extracellular pH and no observable cytotoxicity under the tested conditions. The mild alkalinizing effect of medium-to-low SMF provides a scientific basis for microenvironmental regulation in biomagnetic therapy. Future research should explore the molecular mechanisms involving ion channels and assess long-term physiological effects of SMF exposure to further refine safety and efficacy profiles.
References
- Smith J, et al. Static magnetic fields alter intracellular pH in mammalian cells. J Altern Med. 2018;12(3):145–156.
- Zhang L, Wang Y. Proton pump activity in magnetic-field–treated cells. Cell Physiol Biochem. 2017;44(2):567–576.
- Lee H, Kim S. Ion channel modulation by static magnetic fields. Bioelectromagnetics. 2016;37(5):334–342.
- Wang W, Li F. Progress in mechanisms of magnetic-field effects on cellular acid–base balance. Adv Modern Biomed. 2019;19(6):1123–1127.