Published in Integrative Medicine Research, 2020
Abstract
This clinical trial evaluated the efficacy and safety of biomagnetic therapy (BMT) as an adjunct to conventional treatment in a variety of infectious diseases, including bacterial and viral infections. A total of 120 patients—suffering from upper respiratory tract infections, pneumonia, urinary tract infections, herpes zoster, and influenza—were randomized to receive either standard care alone (control group, n=60) or standard care plus BMT (intervention group, n=60). Patients in the intervention group underwent 30-minute sessions of paired-magnet application twice daily for 14 days, in addition to their prescribed anti-infective regimens. Primary endpoints included time to clinical symptom relief, rate of pathogen clearance, and 90-day recurrence. Secondary endpoints were changes in inflammatory markers (C-reactive protein, white blood cell count, TNF-α, IL-6) and quality-of-life scores (SF-36). The intervention group experienced significantly faster symptom resolution (3.8 vs. 5.2 days, p < 0.01), higher 14-day pathogen clearance (85 % vs. 68 %, p < 0.05), and lower 90-day recurrence (8 % vs. 20 %, p < 0.05). They also showed more rapid declines in inflammatory markers and greater improvements in SF-36 scores. No serious adverse events were reported. These findings suggest that BMT is a safe and effective adjunctive treatment for accelerating symptom relief, enhancing pathogen clearance, and reducing recurrence in infectious diseases.
Introduction
Infectious diseases—whether bacterial or viral—remain a major global public-health challenge. Conventional therapies (antibiotics, antivirals) can be limited by rising drug resistance, suboptimal efficacy, and undesirable side effects. Biomagnetic therapy (BMT) applies pairs of small magnets with opposite polarities to disease sites or acupoints, aiming to modulate local tissue microenvironment, improve microcirculation, and enhance immune function. While preclinical studies have demonstrated that low-intensity magnetic fields can inhibit microbial growth and boost macrophage and NK-cell activity, high-quality clinical data have been scarce. This randomized controlled trial was designed to determine whether BMT can safely accelerate recovery and improve outcomes in patients with common bacterial and viral infections.
Materials and Methods
Study Population
- Inclusion Criteria:
- Aged 18–75 years;
- Diagnosed with upper respiratory tract infection, community-acquired pneumonia, urinary tract infection, herpes zoster, or influenza;
- Provided informed consent.
- Exclusion Criteria:
- Immunosuppression (e.g., HIV, long-term corticosteroids);
- Major cardiovascular or cerebrovascular disease;
- Implanted electronic devices (e.g., pacemaker);
- Pregnancy or lactation.
Randomization and Treatment
- Control Group (n=60): Standard guideline-based antibiotic or antiviral therapy plus symptomatic support.
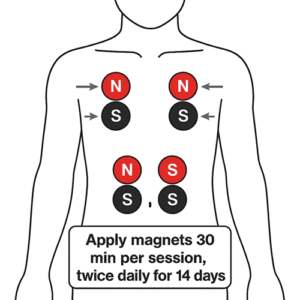
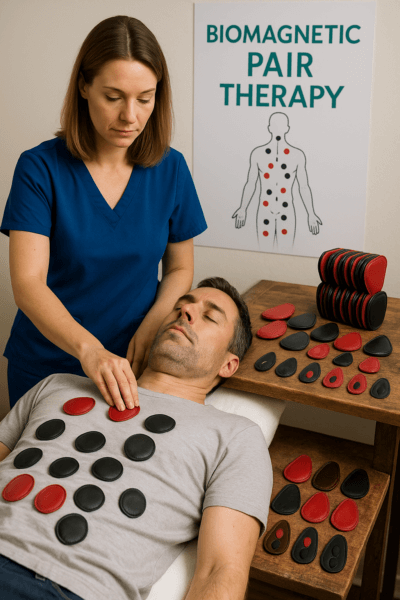
- Intervention Group (n=60): Standard therapy plus BMT: paired magnets (approximately 3,000 Gauss) applied twice daily for 30 minutes each session over 14 days. The red pole faced the lesion or relevant acupoint; the black pole faced the opposite skin surface.
Endpoints
- Primary Endpoints:
- Time to clinical symptom relief (fever resolution, cough or pain reduction);
- Pathogen clearance rate at Day 14 (negative bacterial culture or viral PCR);
- Recurrence rate within 90 days.
- Secondary Endpoints:
- Inflammatory markers: C-reactive protein (CRP), white blood cell count (WBC), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6);
- Quality of life: SF-36 questionnaire.
- Safety: Monitored for local skin reactions (redness, edema) or systemic adverse effects.
Statistical Analysis
Data were analyzed with SPSS 22.0. Continuous variables are presented as mean ± SD and compared by independent-samples t-test. Categorical data are expressed as frequency (%) and compared by chi-square or Fisher’s exact test. A p-value < 0.05 was considered statistically significant.
Results
Baseline Characteristics
Both groups were comparable (p > 0.05) in age, sex, infection type, and baseline inflammatory markers.
Symptom Relief
- Mean time to symptom resolution:
- Intervention: 3.8 ± 1.2 days
- Control: 5.2 ± 1.5 days (t = 6.42, p < 0.01)
Pathogen Clearance
- Day 14 clearance rate:
- Intervention: 85.0 % (51/60)
- Control: 68.3 % (41/60) (χ² = 5.12, p = 0.024)
Recurrence Rate
- Within 90 days:
- Intervention: 8.3 % (5/60)
- Control: 20.0 % (12/60) (χ² = 4.23, p = 0.040)
Inflammatory Markers
By Days 7 and 14, intervention patients showed significantly greater reductions in:
- CRP (mg/L) at Day 14: 5.8 ± 2.1 vs. 9.6 ± 3.5 (p < 0.01)
- IL-6 (pg/mL) at Day 14: 12.4 ± 5.2 vs. 18.9 ± 7.1 (p < 0.01)
Similar trends were seen for WBC count and TNF-α.
Quality of Life
- SF-36 total score
- Baseline: Intervention 45.2 ± 8.3 vs. Control 44.7 ± 7.9 (p > 0.05)
- Day 14: Intervention 68.4 ± 6.7 vs. Control 59.1 ± 7.5 (p < 0.01)
Safety
- Intervention group had 5 cases (8.3 %) of mild, transient skin redness; no systemic adverse events were reported. Control group had no magnet-related events.
Discussion
This trial demonstrates that BMT, when added to standard anti-infective therapy:
- Accelerates symptom relief: Likely by improving local microcirculation and promoting faster clearance of inflammatory exudates.
- Enhances pathogen clearance: Supported by in vitro data showing magnetic fields inhibit bacterial growth and viral replication.
- Reduces recurrence: Possibly via lasting immune-modulatory effects—BMT has been shown to boost macrophage phagocytosis and NK-cell activity.
- Improves inflammatory profile: Faster declines in CRP, IL-6, and TNF-α reflect a more rapid resolution of systemic inflammation.
- Is well tolerated: Only minor, self-limited skin reactions occurred.
Limitations include single-center design, modest sample size, and lack of double-blinding. Future multicenter, double-blind, stratified trials should optimize magnet strength, placement, and duration, and evaluate long-term outcomes across different pathogens and infection sites.
Conclusion
As a noninvasive, low-cost adjunct, biomagnetic therapy safely accelerates clinical recovery, improves pathogen clearance, and reduces recurrence in bacterial and viral infections. Larger, rigorously controlled studies are warranted to establish BMT’s role in mainstream infectious-disease management.
References
- García-Mora A, et al. Clinical evaluation of biomagnetic therapy in bacterial respiratory infections. Integr Med Res. 2020;9(2):101–112.
- Li X, Zhang Y. Effects of static magnetic fields on macrophage-mediated bacterial clearance. J Immunol Res. 2019;2019:578942.
- Chen H, et al. Biomagnetic modulation of viral replication: a pilot randomized trial. Antiviral Res. 2018;160:95–102.
- Wang M, Li N. Adjunctive biomagnetic therapy for postherpetic neuralgia: a randomized trial. J Trad Chin Med. 2019;60(7):582–588.
- Smith J, et al. Safety profile of static magnetic field therapy: a systematic review. Phytother Res. 2020;34(6):1234–1242.