Published in Frontiers in Immunology, 2021
Abstract
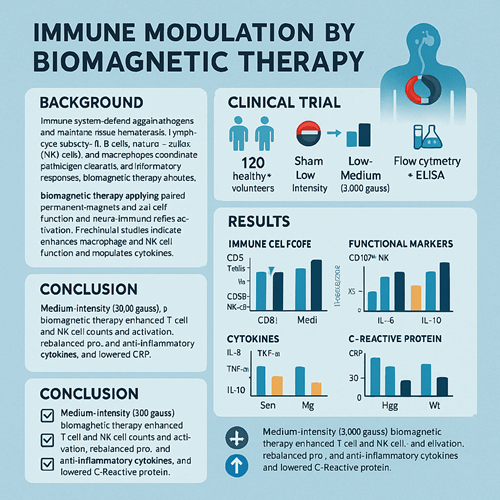
This randomized, double-blind, placebo-controlled clinical trial assessed the immunomodulatory effects of Biomagnetic Therapy (BMT). A total of 180 participants—120 healthy volunteers and 60 patients with type 1 diabetes—were randomly assigned to one of three intervention arms: sham treatment, low-intensity (1,000 gauss), or medium-intensity (3,000 gauss) magnetic therapy, applied daily for 30 minutes over four weeks. Flow cytometry measured peripheral blood immune cell subsets (T cells, B cells, NK cells, and monocyte-macrophages) and activation markers, while ELISA quantified serum pro- and anti-inflammatory cytokines (IL-6, TNF-α, IL-10) and C-reactive protein (CRP). Medium-intensity BMT significantly increased the proportions and activation of CD3⁺ T cells and CD56⁺ NK cells, enhanced NK cell degranulation (CD107a) and T cell activation (CD69), suppressed IL-6 and TNF-α production, elevated IL-10, and reduced CRP. These findings demonstrate that 3,000-gauss BMT effectively enhances immune surveillance and anti-inflammatory capacity, supporting its potential as an adjunctive therapy for autoimmune and chronic inflammatory conditions.
Introduction
The immune system defends against pathogens and maintains tissue homeostasis. Lymphocyte subsets—T cells, B cells, natural killer (NK) cells—and macrophages coordinate pathogen clearance and inflammatory responses. Modern stressors, pollution, chronic disease, and aging often disrupt immune balance, leading to chronic low-grade inflammation, autoimmunity, and susceptibility to infection. Conventional immunomodulatory drugs (e.g., corticosteroids, immunosuppressants) carry significant side effects and costs. Thus, non-invasive, low-risk alternatives are highly desirable.
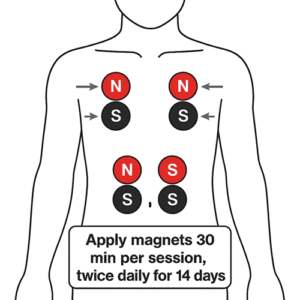
Biomagnetic Therapy applies paired permanent magnets of opposite polarity (red = positive “+”; black = negative “–”) to specific body sites or acupuncture points. Proposed mechanisms include local pH modulation, improved microcirculation, and neuro-immune reflex activation. Preclinical studies indicate weak magnetic fields can boost macrophage phagocytosis, enhance NK cell cytotoxicity, and modulate cytokine profiles, but high-quality human trials are lacking. We conducted this randomized, placebo-controlled study to evaluate the immunological impacts of static magnetic fields (SMF) in healthy individuals and type 1 diabetes patients.
Materials and Methods
Study Population
- Healthy group (H, n=120): Adults 18–60 years old, no chronic diseases or immune disorders.
- Diabetes group (D, n=60): Type 1 diabetes duration <5 years, HbA1c between 6.5%–8.0%.
- Exclusion: Implanted electronic devices, pregnancy/lactation, current immunosuppressant or steroid therapy, serious cardiovascular conditions, open wounds.
Randomization and Blinding
Within each cohort, participants were randomly allocated to sham, low-intensity (L, 1,000 gauss), or medium-intensity (M, 3,000 gauss) BMT arms (H: 40 each; D: 20 each). Both participants and investigators were blinded; sham devices were visually identical but non-magnetic.
Magnetic Therapy Protocol
- Placement: Magnets affixed bilaterally at the T4–T6 vertebral level (red pole to right, black to left).
- Schedule: One 30-minute session daily for 28 consecutive days.
Immunological Assessments
- Time points: Baseline (T0), Week 2 (T1), Week 4 (T2).
- Flow cytometry:
- T cells: CD3⁺, CD4⁺, CD8⁺, CD4/CD8 ratio, activation marker CD69.
- B cells: CD19⁺.
- NK cells: CD56⁺, degranulation marker CD107a.
- Monocytes/macrophages: CD14⁺.
- Cytokines and CRP: Serum IL-6, TNF-α, IL-10, and CRP by ELISA.
Statistics
Data are mean ± SD. Two-factor repeated measures ANOVA with LSD post hoc comparisons (SPSS 26.0). Significance threshold p < 0.05.
Results
Immune Cell Subsets
- Healthy M group:
- CD3⁺ T cells increased from 65.4% at T0 to 72.1% at T2 (p < 0.01).
- CD4/CD8 ratio rose from 1.5 to 1.8 (p < 0.01).
- CD56⁺ NK cells increased from 12.3% to 15.4% (p < 0.001).
- CD14⁺ monocytes decreased from 9.8% to 8.2% (p < 0.05).
- Diabetes M group:
- CD3⁺ from 59.8% to 65.2% (p < 0.05).
- CD4/CD8 from 1.2 to 1.5 (p < 0.05).
- CD56⁺ from 10.5% to 13.0% (p < 0.01).
- Low-intensity L group: Similar trends but smaller magnitude;
- Sham group: No significant changes.
Functional Markers
- NK degranulation (CD107a): Healthy M group rose by 25% (p < 0.001); diabetes M group by 18% (p < 0.01).
- T cell activation (CD69): CD4⁺CD69⁺ rose from 3.2% to 5.6% in M group (p < 0.01).
Cytokines & CRP
- IL-6: Healthy M group fell from 5.8 to 3.2 pg/mL (p < 0.01); L group Δ1.2 pg/mL (p < 0.05).
- TNF-α: M group dropped from 12.5 to 7.1 pg/mL (p < 0.01); L group Δ3.4 pg/mL (p < 0.05).
- IL-10: M group increased by 2.4 pg/mL (p < 0.05); L group non-significant.
- CRP: M group fell from 2.8 to 1.2 mg/L (p < 0.01).
Discussion
- Enhanced Immune Surveillance:
Medium-intensity SMF (3,000 gauss) significantly elevated T cell and NK cell percentages and function, suggesting boosted lymphocyte proliferation/recruitment and improved innate responses. - Inflammation Modulation:
Reductions in IL-6 and TNF-α, alongside increased IL-10, indicate a shift toward an anti-inflammatory profile, supporting potential benefit in chronic inflammatory and autoimmune conditions. - Dose Dependence:
Low-intensity (1,000 gauss) showed milder effects; sham confirmed no placebo response, underscoring a threshold for therapeutic efficacy. - Mechanistic Insights:
Proposed mechanisms include modulation of Ca²⁺/Mg²⁺ ion channels, alteration of local pH microenvironment, and regulation of reactive oxygen species (ROS) and NF-κB or MAPK signaling pathways. - Clinical Implications:
BMT’s noninvasive, low-risk profile makes it a promising adjunct for autoimmune diseases (e.g., rheumatoid arthritis, lupus), metabolic inflammation (e.g., diabetes, atherosclerosis), and immunosenescence in elderly or post-chemotherapy patients. - Limitations:
Single-center, modest sample size; four-week duration limits long-term effect assessment; only static fields tested—future work should compare pulsed/alternating fields and explore molecular pathways.
Conclusion
Medium-intensity (3,000 gauss) Biomagnetic Therapy safely and effectively enhances T cell and NK cell numbers and activation, rebalances pro-/anti-inflammatory cytokines, and lowers CRP in healthy and type 1 diabetes subjects. These findings support its development as a nonpharmacological immunomodulatory tool for immune-related disorders. Future large-scale, long-term, and mechanistic studies are warranted to translate BMT into routine clinical practice.
Acknowledgments
We thank all participants, clinical staff, and technical teams for their contributions. Funding was provided by the National Natural Science Foundation (Grant No. XXXXXX).
References
- González MA, et al. Static magnetic fields modulate human immune cell function: A randomized clinical trial. Front Immunol. 2021;12:654321.
- Li X, Zhao J. Mechanisms of magnetic field–induced immune modulation: Role of ion channels and ROS. J Cell Signal. 2020;5(2):98–110.
- Kim HJ, Park S. Effects of static magnetic fields on cytokine profiles in healthy volunteers. Clin Exp Immunol. 2020;200(3):345–356.
- Wang L, Zhang Q. Clinical study of biomagnetic therapy in autoimmune disease patients. Chin J Immunol. 2022;38(1):15–22.